Abstract
Background: A subset of patients with acute and chronic leukemia have received more than one allogeneic hematopoietic stem cell transplant (alloSCT) to achieve a durable remission. A very small proportion of these patients have received a third alloSCT (alloSCT3), and very little is known about their outcome.
Methods: We identified 18 patients with acute or chronic leukemia who had relapsed or failed to engraft after their second alloSCT, and received alloSCT3 at our institution. Primary objectives were to evaluate relapse-free (RFS) and overall survival (OS). RFS was the time from SCT3 to relapse, death or censored at last follow up date. OS was defined as the time from alloSCT3 to death from any cause or censored at last follow up.
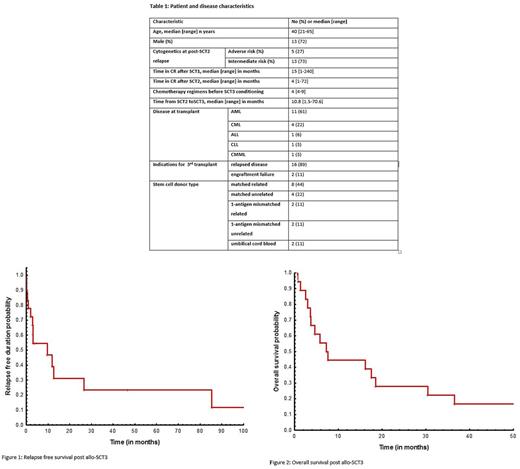
Results: Eleven of the 18 patients, 11 (61%) had acute myeloid leukemia (AML). Patient characteristics are listed in Table 1. Sixteen (18%) patients were considered for SCT3 after disease relapse and 2 (11%) for SCT2 engraftment failure. Five of 11 (55%) AML patients had adverse risk cytogenetics prior to alloSCT3. In the entire cohort (n=18), seven (39%) were in complete remission (CR) at alloSCT3, and 6 patients with AML had active disease with a median bone marrow blast % of 16 [range, 7-40]. Neutrophil and platelet engraftment occurred in 94% and 83% of the cases, respectively. The most common preparative regimen was fludarabine + melphalan that was used in 10 (55%) patients. Other preparative regimens used were fludarabine + cyclophosphamide + rituximab, 2 (12%); decitabine, 2 (12%); others, 4 (23%). Ten (55%) alloSCT3 were from matched-related donors, 6 (33%) from matched-unrelated and 2 (11%) from umbilical cord blood. Median time for neutrophil and platelet count recovery was 18 and 21 days, respectively. Thirteen (72%) patients had a different donor from SCT2 to alloSCT3. Acute and chronic GVHD occurred in 3 (19%) and 5 (31%) of 16 evaluable patients, respectively. Nine of 15 (60%) evaluable patients relapsed after alloSCT3. Two patients, both with AML, experienced delayed relapses at extramedullary sites (breast at 9.5 months; skin at 11.5 months). At a median follow up of 7.5 (range, 0.8-678) months, one-year RFS and OS probabilities were 39% (Figure 1) and 38% (Figure 2), respectively. Both patients transplanted with umbilical cord blood graft experienced short remission durations lasting < 3 months from the receipt of transplant. Two patients (CML 1, CLL 1) are still alive and in remission, beyond 5 years post-alloSCT3.
Conclusions: Our study results suggest that alloSCT3 is a feasible option in a small subset of patients with leukemia and can offer durable remissions in selected cases.
Pemmaraju: novartis: Consultancy, Honoraria, Research Funding; affymetrix: Research Funding; abbvie: Research Funding; stemline: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria; roche diagnostics: Consultancy, Honoraria; Incyte Corporation: Consultancy, Honoraria; cellectis: Research Funding. Kantarjian: Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Novartis: Research Funding. De Lima: Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Cortes: ImmunoGen: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal